Dr. Brian C. Ring
Contact InformationE-Mail: Brian Ring Education & ExperienceB.S. Biological Sciences, Florida State University |
|
TeachingBIOL 1100: Biology Freshmen Seminar
BIOL 1107: Principles of Biology I
BIOL 1951H: Honor’s Biology: Cellular Processes
BIOL 4350: Developmental Biology
BIOL 4580: Molecular Genetics
BIOL 4900: Biology Senior Seminar
BIOL 6350: Developmental Biology- Graduate Level
BIOL 6580: Molecular Genetics- Graduate Level
Research Interests
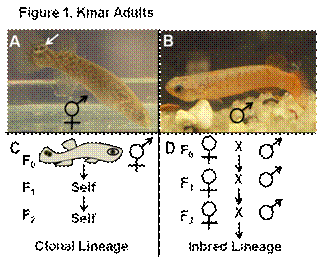 Research in my lab involves molecular genetic techniques toward understanding how the vertebrate gonad develops. Primary sex determination in most vertebrates results in the development of a single gonad, ovary or testis, from a bipotential primordium, whose developmental fate is controlled by genetic or environmental mechanisms or a combination of both. For example, sex type is determined by sex chromosomes (genetics) in mammals (i.e. XY male in humans) or temperature in alligators (environmental). Regardless of mechanism, the predominant result is the formation of dimorphic individuals, either male or female, which have a testis or ovary, respectively. The mangrove killifish, Kryptolebias marmoratus (Kmar) differs from the predominant mode of dimorphic sex determination. Kmar are synchronous self-fertilizing hermaphrodites whose unique form of reproduction involves a mixed gonad structure referred to as an ovotestis (testis and ovaries form in the same place). The ovotestis is capable of normal gametogenesis and fertilization within a common lumen. Most Kmar are configured this way and can easily be self-crossed through several generations to genetic isogeny where they form “clonal lineages” (Fig. 1A & C). Kmar males are rare, but easily distinguishable from hermaphrodites (Fig. 1B). Too date, a pure Kmar female has not been observed in nature or in the laboratory. Kmar fish are analogous to the invertebrate nematode, C. elegans, a hermaphrodite and a well established model organism in developmental genetics. However, sex determination in C. elegans is genetic, but is unknown in Kmar- although temperature does play a role. Both of these model organisms are advantageous for genetic work because the researcher does not have to inbreed males to females to create homozygosity (Fig. 1D).
Research in my lab involves molecular genetic techniques toward understanding how the vertebrate gonad develops. Primary sex determination in most vertebrates results in the development of a single gonad, ovary or testis, from a bipotential primordium, whose developmental fate is controlled by genetic or environmental mechanisms or a combination of both. For example, sex type is determined by sex chromosomes (genetics) in mammals (i.e. XY male in humans) or temperature in alligators (environmental). Regardless of mechanism, the predominant result is the formation of dimorphic individuals, either male or female, which have a testis or ovary, respectively. The mangrove killifish, Kryptolebias marmoratus (Kmar) differs from the predominant mode of dimorphic sex determination. Kmar are synchronous self-fertilizing hermaphrodites whose unique form of reproduction involves a mixed gonad structure referred to as an ovotestis (testis and ovaries form in the same place). The ovotestis is capable of normal gametogenesis and fertilization within a common lumen. Most Kmar are configured this way and can easily be self-crossed through several generations to genetic isogeny where they form “clonal lineages” (Fig. 1A & C). Kmar males are rare, but easily distinguishable from hermaphrodites (Fig. 1B). Too date, a pure Kmar female has not been observed in nature or in the laboratory. Kmar fish are analogous to the invertebrate nematode, C. elegans, a hermaphrodite and a well established model organism in developmental genetics. However, sex determination in C. elegans is genetic, but is unknown in Kmar- although temperature does play a role. Both of these model organisms are advantageous for genetic work because the researcher does not have to inbreed males to females to create homozygosity (Fig. 1D).
Currently, my lab is performing a genetic screen in Kmar for mutations involved in ovotestis development. The hypothesis is that mutants derived from this screen will be sterile by disrupting ovary or testis formation within the mixed ovotestis environment. By comparison of mutants to wild-type individuals of identical clonal decent, a default mechanism is hypothesized that is applicable to understanding the predominant bipotential mode of gonad organogenesis in vertebrates.
Research Sponsored by:

Lab Members:
PRESENT
J. Lance Perry, MS student
J.M. "Angel" Newsome, MS student
Missy Ard, Undergraduate
Lynda Bernhardt, undergraduate
Ginger Moore, undergraduate
PAST
M. Jason Bland, MS Student
Sofia Sucar, undergraduate
Anjelica Askew, undergraduate
Publications
Chiu H, Ring BC, Sorrentino RP, Kalamarz M, Garza D, Govind S.dUbc9 negatively regulates the Toll-NF-kappaB pathways in larval hematopoiesis and drosomycin activation in Drosophila. Dev Biol. 2005, 288(1):60-72.
Department of Biology
-
Room 2035, 2nd Floor
Bailey Science Building -
Mailing Address
1500 N. Patterson St.
Valdosta, GA 31698 - VSU - Department of Biology
- Phone: 229.333.5759
- Department of Biology Fax
- Fax: 229.245.6585
Monday - Friday
8:00 AM - 5:00 PMSaturday - Sunday
CLOSED
